
#Triluma cream Patch
In a patch test study to determine sensitization potential in 221 healthy volunteers, three volunteers developed sensitivity reactions to Tri-Luma Cream or its components. Cutaneous ReactionsĬutaneous hypersensitivity to the active ingredients of Tri-Luma Cream has been reported in the literature. The ACTH or cosyntropin stimulation test may be helpful in evaluating patients for HPA axis suppression. Recovery of HPA axis function generally occurs upon discontinuation of topical corticosteroids. If HPA axis suppression is noted, the use of Tri-Luma Cream should be discontinued. Manifestations of Cushing’s syndrome, hyperglycemia, and glucosuria can also be produced by systemic absorption of topical corticosteroid while on treatment. Systemic absorption of topical corticosteroids can produce reversible hypothalamic-pituitary-adrenal (HPA) axis suppression with the potential for glucocorticosteroid insufficiency after withdrawal of treatment. Tri-Luma Cream contains the corticosteroid fluocinolone acetonide. The majority of patients developing this condition are Black, but it may also occur in Caucasians and Hispanics.
Tri-Luma Cream contains hydroquinone, which may produce exogenous ochronosis, a gradual blue-black darkening of the skin, the occurrence of which should prompt discontinuation of therapy.
Allergic contact dermatitis may also occur. If anaphylaxis, asthma or other clinically significant hypersensitivity reactions occur, institute appropriate therapy and discontinue Tri-Luma. Tri-Luma Cream contains sodium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening asthmatic episodes in susceptible individuals. Warnings and Precautions Hypersensitivity Tri-Luma Cream is contraindicated in individuals with a history of hypersensitivity to this product or any of its components. Related/similar drugs Triderma Dosage Forms and StrengthsĮach gram of Tri-Luma Cream contains 0.1 mg of fluocinolone acetonide, 40 mg of hydroquinone, and 0.5 mg of tretinoin in a light yellow, hydrophilic cream base. It is not for oral, ophthalmic, or intravaginal use. Patients may use moisturizers and/or cosmetics during the day. Therapy should be discontinued when control is achieved.ĭuring the day, use a sunscreen of SPF 30, and wear protective clothing.
#Triluma cream skin
Apply Tri-Luma Cream to the hyperpigmented areas of melasma including about 1/2 inch of normal appearing skin surrounding each lesion. Gently wash the face and neck with a mild cleanser. Tri-Luma Dosage and AdministrationĪpply a thin film of Tri-Luma Cream to the effected area once daily, at least 30 minutes before bedtime. The safety and efficacy of Tri-Luma Cream in the treatment of hyperpigmentation conditions other than melasma of the face have not been studied.īecause pregnant and lactating women were excluded from, and women of childbearing potential had to use birth control measures in the clinical trials, the safety and efficacy of Tri-Luma Cream in pregnant women and nursing mothers have not been established. Excessive bleaching resulting in undesirable cosmetic effect in patients with darker skin cannot be excluded.
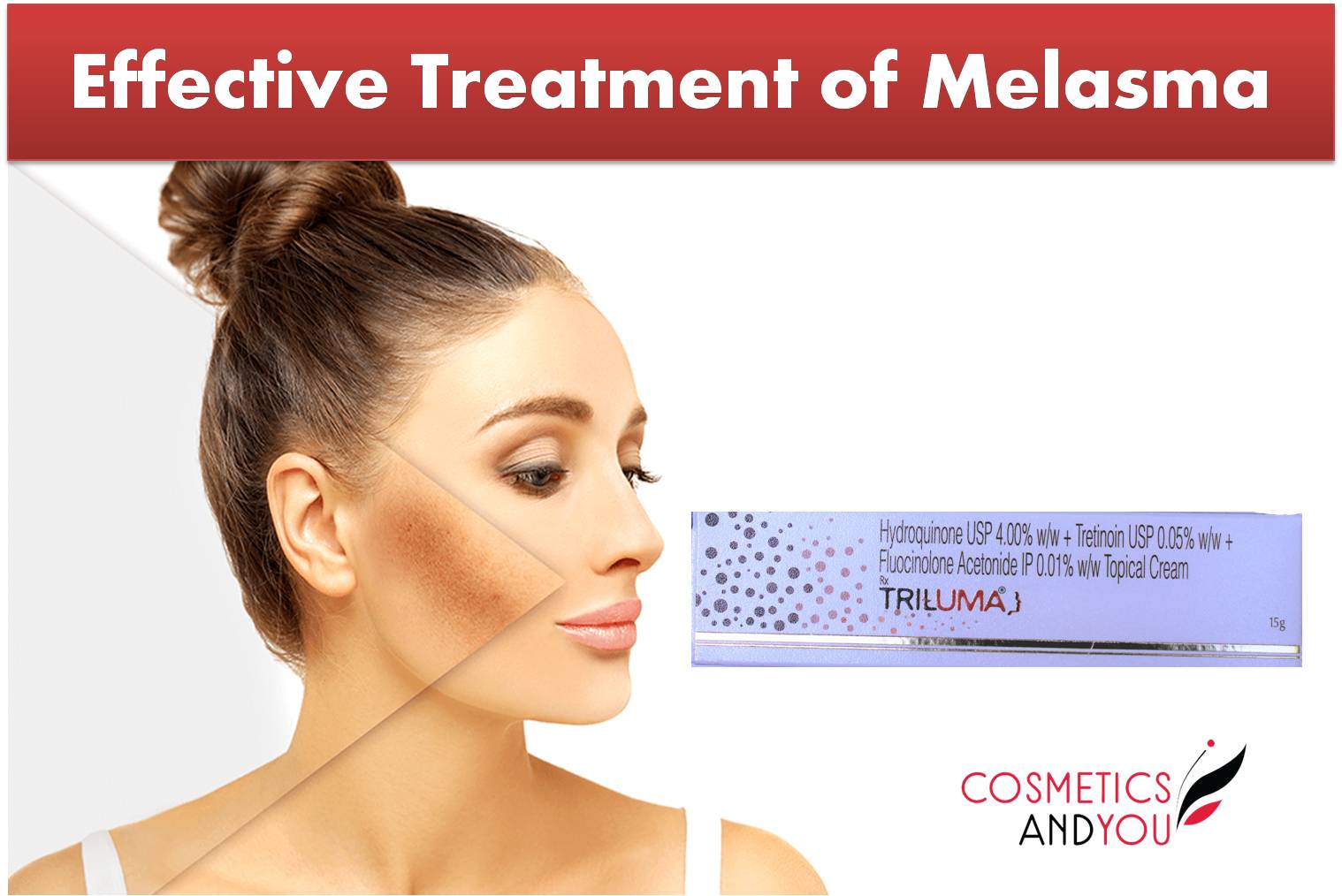
The safety and efficacy of Tri-Luma Cream in patients of Fitzpatrick Skin Types V and VI have not been studied. Melasma usually recurs upon discontinuation of Tri-Luma Cream. After achieving control with Tri-Luma Cream, some patients may be managed with other treatments instead of triple therapy with Tri-Luma Cream. Tri-Luma Cream is NOT indicated for the maintenance treatment of melasma. Tri-Luma Cream is a combination of fluocinolone acetonide (a corticosteroid), hydroquinone (a melanin synthesis inhibitor), and tretinoin (a retinoid) that is indicated for the short-term treatment of moderate to severe melasma of the face, in the presence of measures for sun avoidance, including the use of sunscreens. Expand Indications and Usage for Tri-Luma Indication


 0 kommentar(er)
0 kommentar(er)
